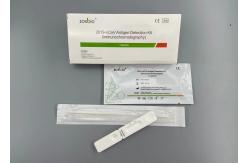
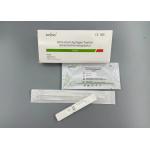
15 Minutes COVID-19 Test Kit Antigen Rapid Test Kit Colloidal Gold
|
2019-NCoVAntigen Detection Kit(Immunochromatography)Intended UseThe product adopts cross-flow immunoassay, it used for the
qualitative detection ofnovel Coronavirus antigen in suspected
patients nasopharyngeal and oropharyngeal swabs. Wide range of applications: suitable for hospitals, disease control centers, communities,airports, stations, customs, schools, enterprises, etc. Sample Requirements
【Sample Requirements】 A polyester sponge sample with a PP (polypropylene) rod is recommended for sterile sample collection. (1) The collection method of oropharyngeal sample: the head of the collected person is slightly tilted, the mouth is open, and the pharyngeal tonsils on both sides are exposed.Apply the swab across the base of the tongue to the pharyngeal tonsils on both sides of the recipient for at least 3 times, and then wipe the swab up and down the posterior pharyngeal wall for at least 3 times. (2) Nasopharyngeal sample collection method: sampling personnel
gently support the head of the collected personnel with one hand,
with one hand holding the swab, the swab is inserted into the
nostril, and slowly penetrate backward along the bottom of the
inferior nasal meatus. Because the nasal meatus is curved,
excessive force should not be used to avoid traumatic bleeding.When
the top of the swab reaches the posterior wall of the nasopharynx,
gently rotate the swab once (pause for a moment in case of reflex
cough), and then slowly remove the swab. (3) Sample treatment: Samples collected should be treated with the sample buffer provided by this kit as soon as possible (if not immediately processed, the samples should be stored immediately in a dry, sterilized and tightly sealed plastic tube), and stored at 2℃ to 8℃ for no more than 24h;Long-term storage at -70℃, but repeated freezing and thawing should be avoided. Test MethodRestore all reagents to room temperature before testing. The test
should be conducted at room temperature. II. Test Procedure (See Figure 2)
Interpretation of test results① Invalid results: no reaction line appeared in the quality control
line (line C), and the test was invalid. The experiment should be
redone.
Company profileZhongxiu Science And Technology Co.,Ltd., is a high-tech enterprise
engaged in the research and development, production and operation
of in vitro diagnostic products. The in vitro diagnostic products
developed by the company cover POCT series, microbial series,
biochemical series and immune series reagents and supporting
instruments. The company has always adhered to the core concept of "fast and accurate, living up to life", committed to providing society with excellent products and services, and contributing to the cause of human health. Zhongxiu QualificationsDOC |
| Product Tags: antigen rapid test kit colloidal gold colloidal gold COVID-19 Test Kit Colloidal Gold 15 minute antibody test |