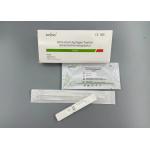
NCoV Rapid COVID-19 Test Kit Immunochromatography Intended Use
|
NCoV Rapid Ag Test Kit Immunochromatography Intended UseIntended UseThis product is used for qualitative detection of nasopharyngeal
swabs and oropharyngeal swabs of patients with suspected infection
by side-flow immunoassay Sample Requirements
[Sample requirements] It is recommended to use polyester sponge samples with PP (polypropylene) rods for sterile sample collection. (1) Oropharyngeal specimen collection method: the collection head is slightly tilted, the mouth is open, and both sides of the pharyngeal tonsil are exposed. Swab along the base of the tongue on both sides of the pharyngeal tonsils at least 3 times, then wipe the posterior pharyngeal wall up and down with a cotton swab at least 3 times. (2) Method of collecting nasopharyngeal specimens: the sampler gently supports the head of the person to be collected with one hand and holds the swab in the other hand. The swab is inserted into the nostril and then slowly penetrated along the bottom of the inferior nasal canal. Arc, do not exert too much force to avoid traumatic bleeding. When the tip of the swab reaches the posterior wall of the nasopharyngeal cavity, gently rotate it once (stay for a while if it is reflexive cough) and slowly remove the swab. (3) Sample processing: The collected samples should be treated with the sample buffer provided by the kit as soon as possible (if not immediately, the samples should be stored in a dry, sterilized, tightly sealed plastic tube) and stored at 2℃ ~ 8℃ for no more than 24 hours; Long-term storage at -70℃, but should avoid repeated freeze-thaw. Test MethodBefore testing, restore all reagents to room temperature. The test
should be carried out at room temperature. Ii. Test Procedure (See Figure 2)
Interpretation of test resultsSchematic diagram of test card result judgment: Company profileZhongxiu QualificationsDOC |
| Product Tags: NCoV Rapid COVID-19 Test Kit Intended Use COVID-19 Test Kit Immunochromatography covid 19 rapid test kit |