Colloidal Gold Based COVID-19 Test Kit For Negative Nucleic Acid Testing
|
Detailed Product Description
2019-NCoV IgM / IgG Detection Kit (Colloidal Gold-Based) Test MethodIntended UseThis product is used to detect novel coronavirus (2019-nCoV) IgM /
IgG antibodies in human serum, plasma or whole blood, as a
supplementary testing index for negative nucleic acid testing or in
collaboration with the diagnosis of nucleic acid testing, This product is to be used as an auxiliary diagnosis and emergency
reserve for pneumonia patient infected by 2019-nCoV Test MethodPrepare before the 1. experiment Remove the kit and the samples to be tested, and restore all the reagents and samples to room temperature 2. Experimental Steps ① opens the testing reagent package, removes the test card, and
puts it flat. ② adds the sample 10 μL, in the detection card and then adds 2 drops (about 60 μL) sample buffer in the sample hole. ③ The room temperature reaction was observed for 10~15 minutes, and was invalid after 20 minutes. ④ The concise operator opens the package, removes the detection card, and lays it flat ↓ The sample to be examined 10 μL is added to the sample end of the
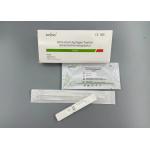
testing reagent ↓ Add 2 drops (about 60 μL) sample buffer to the sample hole ↓Room temperature reaction was 10 ~ and 15 minutes Visual judgment reading results Interpretation of test resultsNegative,Positive,Invalid
Judgment figure of the test card result shows: ① Invalid Result: No visible red line or only the detection line (T line) and no quality control line (C line), the detection is invalid, and the test should be renewed. ② negative results: A red mass control line (C line) indicating no antibodies in the sample or below the detection level. ③ positive results: two red lines (one red QC line and one of the red tests) or three red lines (one red QC line and two red test lines). Company profileZhongxiu Science And Technology Co.,Ltd., is a high-tech enterprise
engaged in the research and development, production and operation
of in vitro diagnostic products. The in vitro diagnostic products
developed by the company cover POCT series, microbial series,
biochemical series and immune series reagents and supporting
instruments. The company has always adhered to the core concept of "fast and accurate, living up to life", committed to providing society with excellent products and services, and contributing to the cause of human health. Zhongxiu QualificationsDOCWhite List
|
| Product Tags: Colloidal Gold Based COVID-19 Test Kit Negative Nucleic COVID-19 Test Kit Negative Nucleic colloidal gold rapid test |
Related Products
Email to this supplier