TUV Home Use Colloidal Gold CIA Treponema Pallidum TP Syphilis Rapid Test Kit
|
|
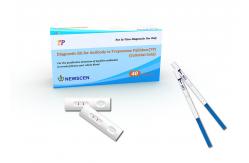
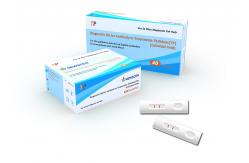
Treponema Pallidum(TP) Rapid Diagnostic Kit (Colloidal Gold)
For the qualitative detection of Syphilis antibodies in serum/plasma and whole blood
TP (Treponema Pallidum) Syphilis Antibody Rapid Test Kit In Vitro Test Fast Detection Cassette Home Use
Intended Use
Principle
Storage
Assay Procedure
Serum or Plasma Sample Add 2 drops of serum or plasma into sample well. Observe the result in 120 minutes.
Whole Blood Sample Add 1 drop of whole blood into sample well, after all blood completely absorbed. Add 2 drop of whole blood diluent. Observe the result in 20 minutes.
Interpretation of Results Negative:
No apparent band in the test region (T), Only one red band appears in the control region (C). This indicates that no TP antibodies have been detected.
In addition to one red band in the control region (C), a red band will appear in the test region (T). This indicates that the specimen contains TP antibodies.
If no band appears in the control region(C), regardless of the
presence or absence of band in the test region (T). It indicates a
possible error in performing the test. The test should be repeated
using a new device.
|
| Product Tags: CIA TP Syphilis Rapid Test Kit TUV TP Syphilis Rapid Test Kit Treponema Pallidum Syphilis Test Kit |