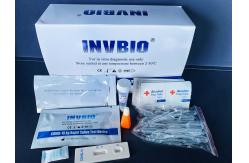
Covid 19 Self Neutralizing Antibodies Test Kit In Vitro Diagnostic
|
|
COVID-19 Neutralizing Antibody Rapid Test Device CE FCS certificate Covid 19 Serum Plasma Whole Blood Neutralizing
Antibody Rapid Test Kit INTENDED USE CE mark Coronavirus (SARS-Cov-2) COVID-19 Neutralizing Antibody
Rapid Test Device The COVID-19Neutralizing Antibody Rapid Test is a lateral flow chromatographic immunoassay for
qualitative detection of neutralizing antibody to SARS-CoV-2 in human whole blood,serum,or plasma as an aid in the evaluation of human anti- SARS-CoV-2
neutralizing antibody titer.
DIRECTIONS FOR USE Allow the test device,specimen,buffer,and/or controls to reach
room temperature (15-30°C) prior to testing. 1. Bring the pouch to room temperature before opening.Remove the test device from the sealed pouch and use it as soon as possible. 2. Place the test device on a clean and level surface. 3. Add 20μl whole blood or 10μl serum and plasma into the sample well of the test device, then add 80μl buffer into the sample well. 4. Read the result at 15~20 minutes. The result is invalid after 20 minutes. Note: 1 drop volume of the disposable plastic pipette provided is about 10μl. INTERPRETATION OF RESULTS NEGATIVE:Presence of a single colored band in the control region (C) indicates the absence of COVID-19 neutralizing antibodies or that the concentration of antibodies in sample is below the detection cut-off level. POSITIVE: Presence of two visible, pink-colored bands, one in the control region (C) and one in test region (T), indicates presence of COVID-19neutralizingantibodies in sample. INVALID: There is no line appeared in the C region. Insufficient buffer volume or incorrect procedural techniques are the most likely reasons for control line failure.Review and repeat the procedure with a new test device. If the problem persists,discontinue using the test kit immediately and contact your local distributor. Company Information Innovation Biotech (Beijing) Co., Ltd. is a biotechnology company specializing in research, development and manufacturing of advanced medical in-vitro diagnostic (IVD) rapid test kits, medical and laboratory disposal products. We provide one step medical diagnostic rapid test kit based on our INVBIO brand, Product include Fertility Tests, Tumor markers, DOA drug of abuse test, Drug test cup, Urinalysis reagent strip, ELISA kit, Digital alcohol tester, urine analyzer, et. OEM packaging is available, drug of abuse test, troponin I test, alcohol screening saliva test strips, urine strips, elisa kits, microscope slides Our focus is to expand our markets internationally by forming strategic alliances and entering into partnerships with distributors worldwide. We have established an international reputation for excellence in the manufacturing of quality medical and laboratory products. In addition, we have obtained approval licenses ISO13485, FSC certificate, and most of our products get CE mark Our objective is the utmost satisfaction of our clients all around the world by supplying our quality and economical products
Certifications FAQ Q: What is your payment terms? A: Payment terms: 100% TT before shipment Q: Can you send samples? A: Yes,SAMPLES can be sent for your evaluation.customer pay the freight charge Q: Do you give any discount?
|
||||||||||||||||||
| Product Tags: Covid 19 Neutralizing Antibodies Test Kit In Vitro self antibody test kit In Vitro Neutralizing Antibodies Test Kit |
|
Home CE Approved Igg Igm Antibody Test Kit SARS-CoV-2 Serum Plasma 25pcs/ Box |
|
Ce Covid-19 Test Rapid Igg Igm Antibody Diagnostic Box Of 25 Tests |
|
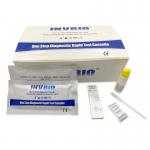
FDA CE Approved Covid 19 Rapid Test Card IgG IgM Antibody Home Kit |
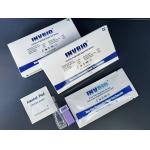
|
SARS-CoV-2 Coronavirus IgG IgM Antibody Test Kit One Step Self Testing INVBIO |
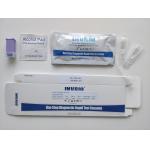
|
CE Antibody Rapid Test Kit Covid-19 Sars Igm / Igm Ab Device |
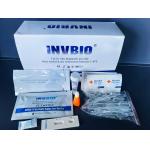
|
Covid 19 Self Neutralizing Antibodies Test Kit In Vitro Diagnostic |