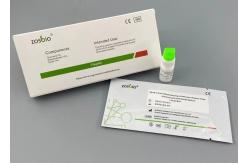
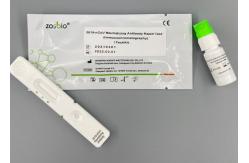
COVID-19 Neutralizing Ag Rapid Test Kit Immunochromatography
|
(COVID-19) Neutralizing Antibody Rapid Test (Immunochromatography) Interpretation Of Test Results
Intended UseThe kit is used for the qualitative detection of neutralization in
2019-nCoV Principle of Detection
Kkit use immunotomography. The test card contains the quality
control line C, test line T and the reference line r, sample to
test (serum / plasma / whole blood) spread upward through capillary
action at the loading hole, combining the S-RBD in the pad to the
fixed ACE2 protein on the NC film, and the signal can be detected
at the test line position. If neutralizing antibodies in the
sample, it binds to the labeled S-RBD antigen as it flows through
the marker pad. Neutralizing antibodies prevent S-RBD from binding
to ACE2, resulting in decreasing signal values, the T line signal
values negatively correlated with neutralizing antibody content,
and no color when the neutralization antibody concentration is high
enough. For the T line, the T line are colored. Quality control
line are used for quality control. If there is no color display on
the C line, the test is not valid and the sample must be retested. Main ComponentsThe kit consists of test cards and a sample buffer.
Storage Conditions and ValidityHold at 2 ℃ ~ 30 ℃ and valid is set to 12 months. The unpacking period of the aluminum foil package is valid for one hour. Batch number: See the label for details. Sample Requirements1. collected serum, plasma, or whole blood samples. Test MethodRead the instructions carefully before testing. Return all reagents
to room temperature at room temperature. Interpretation of Test Results
Figure for judging test card results: 1.Invalid result: quality control line (C) is not reactive and
should be retest. Limitation of Test Method
1. The kit is used for qualitative detection and only for in vitro
assisted diagnosis. Product Performance Indicators 1. Analysis of specificity 1.1 Cross reaction: evaluate the interference of the following type of antibody to the reagent and show no cross reaction.
1.2 Interference substances: In 2019-nCoV and in the Ab test program, add the following concentration to the sample to assess its potential interference. The results show that the various interfering substances do not disturb the detection results of the reagent.
2. Clinical study: When the marketed 2019-nCoV IgG Ab test reagent (colloidal gold method) was used as the control reagent, 120 positive samples and 300 negative samples were selected for testing. The results are summarized as follows:
Precautions1. This product is used for in vitro diagnosis only. Logo interpretation
Basic InformationZHONGXIU SCIENCE AND TECHNOLOGY CO.,LTD Dingluan industrial zone ,Changyuan City,453400,P.R.CHINA Tel:+86-371-55016575 Email:zosbio@zosbio.com Web:www.zosbio.com SUNGO Europe B.V. Olympisch Stadion 24, 1076DE Amsterdam, Netherlands
Company profileZhongxiu Science And Technology Co.,Ltd., is a high-tech enterprise
engaged in the research and development, production and operation
of in vitro diagnostic products. The in vitro diagnostic products
developed by the company cover POCT series, microbial series,
biochemical series and immune series reagents and supporting
instruments. The company has always adhered to the core concept of "fast and accurate, living up to life", committed to providing society with excellent products and services, and contributing to the cause of human health.
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Product Tags: Neutralizing Ag Rapid Test Kit COVID-19 rapid antigen test kit Ag Rapid Test Kit Immunochromatography | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||

|
Disposable Green Blood Collecting Vessel |
|
22G Venous Blood Collection Needle |

|
0.85mm/1.75mm Heel Incision Device |

|
2019 NCoV IgM IgG Rapid Test Antigen Rapid Test Kit Colloidal Gold |

|
96.67% Sensitivity Ag Rapid Test Kit Lateral Flow Immunochromatography |

|
15 Minutes Antibody Fast Detection Kit 98.33% Specificity |