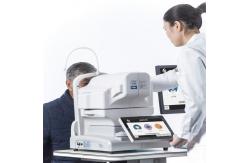
Ophthalmic Digital Fundus SLR Camera One Key Click 135° Wide Field
|
|
ophthalmic fundus camera SLR camera one-key click 135° wide field
Digital Fundus Camera RetiCam 3100 can be used to capture the anterior and posterior eye images. It has a field of view of 50 degrees for fundus imaging.Digital Fundus Camera Reticam 3100 is a highly recognized and popular automatic fundus imaging system around the world. Through dual camera imaging, image control and feature recognition, eye XYZ 3D automatic positioning is realized. Through CCD imaging feedback, the exposure is measured, and the exposure intensity is automatically and accurately determined, without the need for a doctor's complex operation. By controlling the focusing step length according to the image resolution, the image definition can be automatically adjusted to obtain a clear and accurate fundus image and help doctors make accurate judgments for patients.
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Product Tags: Ophthalmic Fundus Camera 135° Wide Field Fundus Camera Digital Fundus SLR Camera | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||

|
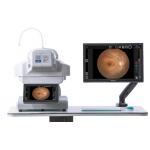
High Resolution Digital Fundus Camera Minimum Pupil 3.3mm Red Free (FFA)/(FAF) |
|
Highly Accurate Retinal Fundus Camera And Easy To Use Digital Fundus Camera |

|
Digital Fundus Photography Camera Machine 35mm Effective Management Of Results |

|
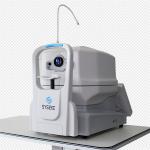
Ophthalmic Digital Fundus Camera Optic Ultra High Resolution Fully Auto |
|
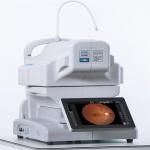
Multifunctional Digital Fundus Camera Photography Equipment Professional Grade |
|
Ultra Wide Field Digital Fundus Camera Dual System Minimum Pupil Size Of 3.3mm |