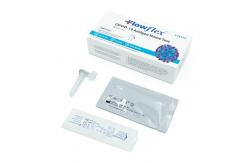
FDA TGA Nasopharyngeal Antigen Test Flowflex Nasal Specimen
|
Detailed Product Description
FDA TGA Nasopharyngeal Antigen Test Flowflex Nasal SpecimenA rapid test for the detection of SARS-CoV-2 antigens in anterior
nasal specimens directly from individuals within 7 days of symptom
onset or without symptoms or other epidemiological reasons to
suspect COVID-19 infection. Materials Provided: •Test Cassette(s) •Package Insert •Extraction Buffer Tube(s) •Nasal Swab(s) • This product has not been FDA cleared or approved but has been
authorized by FDA under an EUA. • This product has been authorized only for the detection, not for
any other viruses or pathogens. • The emergency use of this product is only authorized for the
duration of the declaration that circumstances exist justifying the
authorization of emergency use of IVDs for detection and/or diagnosis of Clear 19 under Section 564(b)(1)
of the Federal Food, Drug and Cosmetic Act, 21 U.S.C. §
360bbb-3(b)(1), unless the declaration is terminated, or authorization is revoked sooner. • For more information on EUAs please visit:
https://www.fda.gov/emergency-preparednessand-response/mcm-legal-regulatory-and-policy-framework/emergency-use-authorization • For the most up to date information on Clear 19, please visit:
www.cdc.gov/Clear 19 • For detailed instructions, please visit: www.aconlabs.com |
| Product Tags: FDA Covid 19 Rapid Test TGA Covid 19 Rapid Test Nasopharyngeal Flowflex Home Test Kit |
Related Products
|
FDA IVD Antigen Rapid Test Kit Colloidal Gold Immunochromatography |

|
Signo Colloidal Gold Diagnosis Saliva Nasal Swab Ivd Rapid Antigen Testing Kit |

|
Saliva Sputum Self Rapid Antigen Test Kit Nasal Swab Signo IVD Diagnostic |

|
Antigen Rapid Test Kit High Accuracy CE Europe Approval |

|
Sterile SARS-CoV-2 Antigen IVD Kit Oropharyngeal Swab Rapid Antigen Swab Test Kit |

|
15 Minutes Covid 19 Rapid Test Kit Nasal Specimen Nasopharyngeal Test Kit |
Email to this supplier