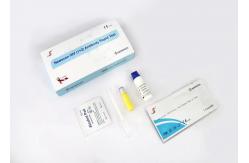
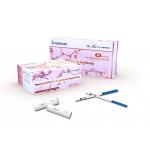
Home One Step Fingertip Specimens 1+2 HIV Rapid Test Kit
|
|
HIV(1+2) Antibody Rapid Test Kit For qualitative detection of HIV(1+2) antibodies in serum/plasma and whole blood
The HIV 1+2 Rapid Test is a single-use rapid device for qualitative detection of antibodies to Human Immunodeficiency Viruses (HIV) in blood, serum and plasma specimens. The test is intended for use in health facilities by trained staff as an aid for the diagnosis of clinical conditions related to infection with HIV-1 and/or HIV-2 – the etiological agents of the acquired immunodeficiency syndrome (AIDS). It provides a clear result within 5-30 minutes.
Interpretation of Results
Negative: No apparent band in the test region (1 and 2), only one red band appears in the control region (C). This indicates that no HIV1/2 antibodies have been detected.
Certification
Our many products are with CE, ISO9001, ISO13485, FDA, FSC, CFDA, Etc. |
| Product Tags: Fingertip Specimens HIV Rapid Test Kit Fingertip Specimens AIDS Rapid Test Kit |