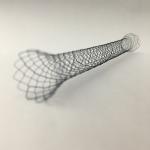
Surgical Nitinol Covered Biliary Stent with CE and ISO certificate
|
|
Surgical Biliary Nitinol covered stent by CE ISO approved
Product Description
Specification : Specification table (for biliary stents )
Usage: Be used to ameliorate the pain caused by biliary stricture and obstruction. The stent is indicated for palliative treatment for patients with bile duct stenosis caused by malignant bile duct carcinoma which cannot undergo surgeries. It is also indicated for stenosis caused by benign tumor which cannot undergo surgeries or the patients are not willing to take surgeries. Its main purpose is to alleviate bile duct obstruction. Characteristics: 1. Continued gentle radial tensile force, effectively expands the stenosis, reducing the patients' discomfort.
2. Imaging markers of the stents are clear visible under image equipment .Positioning is more accurate.
3. Re-positioned introducer system (When releasing less than two-thirds of the length, Stents can be pulled into the introducer system be re-positioned. Separately meets the under endoscope look and image placement and release. Choose the metal mesh grid introducer system. Observe working condition clearly under endoscope. Positioning is more accurate. The code of stent shape:
Stent System Structure Sketch
Our other stent show :
|
|||||||||||||||||||||||||||||||||
| Product Tags: 0.5kg 10mm Biliary Stent Nitinol temporary bile duct stent ISO13485 FN 401 Biliary Stent |
|
|
Silicon Covered Biliary ERCP Stent With Nickel Titanium Shape Memory Alloy Wire |

|
Implant Medical Metal ERCP Biliary Stent Customized In Digestive Tract |
|
|
Non Vascular Nitinol PTCD Biliary Stent Full Silicone Covered With Delivery System |
|
ERCP Biliary Stent Implant Materials Medical Covered And Uncovered Class II |
|
|
Silicon Covered PTCD Bile Duct Stent With Ni Ti Memory Alloy Elastic Wire |

|
CE Certificated ERCP Biliary Stent System Implant Materials & Artificial Organs |


