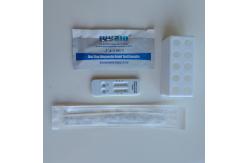
Home Self Testing FSC Rapid Antigen Kit SARS-CoV-2 & Flu A/B
|
|
Home Use Self Testing SARS-CoV-2 & Flu A/B Rapid Antigen Test
COVID-19/Flu A&B test is a lateral flow immunoassay intended
for the in vitro rapid, simultaneous qualitative detection and
differentiation of nucleocapsid antigen from SARS-CoV-2, influenza
A and/or influenza B directly from nasopharyngeal swab specimens
obtained from individuals, who are suspected of respiratory viral
infection consistent with COVID-19 by their healthcare provider,
within the first five days of onset of symptoms. Specification: Sample Type: nasopharyngeal swab specimens Detection Method: Colloidal Gold Detection Time: 15 minutes COVID-19 – Anterior Nasal – Sensitivity 95.45%, Specificity 99.99% • Flu A & B – Sensitivity 99.99%, Specificity 99.99% All necessary reagents provided and no equipment is needed Each Box Contains: 25 individually packed test card 25 Extraction Reagent capsules 25 individually packed sterile Swabs 1 Package Insert /Instructions for use 1 Quick Reference Instructions Date sheet
Directions for Use Allow the test, specimen, extraction buffer to equilibrate to room
temperature (15-30°C) prior to testing. 1. Remove the test device from the sealed foil pouch and use it as
soon as possible. Place the test device on a clean and level surface. Best results
will be obtained if the assay is performed immediately after
opening the foil pouch. 2. Take out one extraction tube with buffer, pull off the sealed
aluminum foil on the extraction tube and place extraction tube in
the tube stand or in the hole in the box. 3. Place the sterilized swab specimen in the sample extraction
buffer. Rotate the swab for approximately 10 seconds while pressing
the head against the inside of the tube to release the antigen in
the swab. 4. Remove the sterilized swab while squeezing the sterilized swab
head against the inside of Buffer as you remove it to expel as much
liquid as possible from the swab. Discard the sterilized swab in
accordance with your biohazard waste disposal protocol. 5. Screw on and tighten the Nozzle with Filter onto the specimen
collection tube, then shake the specimen collection tube vigorously
to mix the specimen and the sample extraction buffer. See
illustration 4. 6. Add 3 drops of the solution (approx.80ul) to each sample well
and then start the timer. Read the result at 15~20 minutes. Don’t
interpret the result after 20 minutes. 7. Please dispose the test materials which put in a closed plastic
bag with the household trash. If there are local regulations,
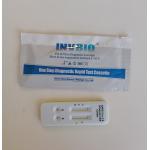
please follow them. 8. Wash hands thoroughly after test completion POSITIVE SARS-Cov-2: Two red lines appear. One red line appears in the control
region(C), and one red line in the test region(T). POSITIVE Influenza A: Two distinct colored lines appear. One colored line should be in
the control region (C) and another colored line should be in the
Influenza A region (A). A positive result in the Influenza A region
indicates that Influenza A antigen was detected in the sample. POSITIVE Influenza B: Two distinct colored lines appear. One colored line should be in
the control region (C) and another colored line should be in the
Influenza B region (B). A positive result in the Influenza B region
indicates that Influenza B antigen was detected in the sample. POSITIVE Influenza A and Influenza B: Three distinct colored lines appear. One colored line should be
in the control region (C) and two colored line should be in the
Influenza A region (A) and Influenza B region (B). A positive
result in the Influenza A region and Influenza B region indicates
that Influenza A antigen and Influenza B antigen were detected in
the sample. *NOTE: The shade of color may vary, but it should be considered
positive whenever there is even a faint line. If the test result is positive, please keep social distant to not
spread infection, to follow local guidelines about social distance. NEGATIVE: Only one red line appears in the control region(C), and no line
in the test region(T/A/B). If you have symptoms like fever, cough and/or shortness of breath.
Please retest in 1-3 days. You must continue following the
applicable hygiene and distancing rules even with a negative
result. INVALID: No red line appears in the control region(C). The test is invalid
even if there is a line on test region(T/A/B). Insufficient sample
volume or incorrect procedural techniques are the most likely
reasons for control line failure. Review the test procedure and
repeat the test using a new test device. Certifications
2022 MEDICA in Germany Date: from November 14th to November 17th Add.: Messe Düsseldorf Hall: 01 Out booth No: 1H39 FAQ Q: What is your payment terms? A: Payment terms: 100% TT before shipment Q: Can you send samples? A: Yes,SAMPLES can be sent for your evaluation.customer pay the freight charge Q: Do you give any discount? |
||||||||||||||||||
| Product Tags: sars-cov-2 rapid antigen kit fsc rapid antigen kit flua/b rapid antigen test at home |
|
Ce Certified Rtk Coronavirus Antigen Swab Test Self Test 24 months |
|
98% Accuracy Ce Certified Rtk Coronavirus Antigen Swab Test Self Test |
|
Convenient Safe Testing With Covid-19 Ag Saliva Rapid Test Kit Lollipop |
|
Accuracy Reliability Covid-19 Oral Fluid Rapid Antigen Test Kit |
|
99.9% Antigen Kit Covid-19 Influenza A/B Viruses Flu A/B Combo Cassette |
|
95.6% Sensitivity Home Covid 19 Rapid Self Test Kit Nasopharyngeal Swab with CE |