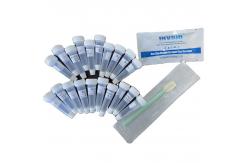
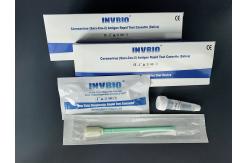
Qualitative Detection Ag Rapid Test Cassette Lollipop Self Rtk Antigen Test
|
|
Qualitative Detection Ag Rapid Test Cassette Lollipop Self Rtk Antigen Test
Product Description For the qualitative detection self test kit Covid 19 Ag saliva
rapid test kit (lollipop) COVID-19 Ag Rapid Saliva Test Device is a rapid
chromatographicimmunoassay for the qualitative detection of N
antigen from SARS-CoV-2 present in human saliva within the first 7
days of symptom onset. This test is for professional used only, as
an aid toearly diagnosis of SARS-CoV-2 infection in patient.The
result of this test should not be the sole basis for the diagnosis;
confifirmatory testing is required Date sheet
Directions for Use Do not place anything into the mouth including food, drink, gum, or
tobacco products for at least 10 minutes prior to collection of saliva specimen. 1.Remove the swab from the sealed pouch. 2.Cough deeply twice before collecting the samples. 3. Insert the sponge end of the collector into the mouth. Then swab
back and forth along the gum line from one end to the other end of the upper and lower gum
for 3 to 5 times. Then slowly scrape the cheek inside the mouth for 3 to 5 times and
slowly scrape the tongue surface for 3 to 5 times.Then put the collection pad on the tongue
for 1 minutes 4.Open the lid of the collection tube. Remove the saturated
collector pad out of the mouth and place it into the tube which contains 1 ml sample extraction
buffer, and then fully stirred along the wall of the tube. Break off the collector,
leaving the sponge end of the collector in the chamber. 5.Tighten the lid of the collection tube and mixing liquid of the
chamber well before using (Shaking up and down for about 20 times). Allow the test, specimen, extraction buffer to equilibrate to room
temperature (15-30°C) prior to testing. 1. Remove the test device from the sealed foil pouch and use it as
soon as possible. Best results will be obtained if the assay is
performed immediately after opening the foil pouch. 2. Put the test device on a clean and level surface. 3. Shake the swab specimen in the extraction vial to well mix. 4. To run test, twist open the bottom screw cap of the extraction
vial to expose dropper tip.Transfer 3 drops (~90μl) of the specimen
to the sample well of the test device and make sure a colored
liquid appearing in the detection window in 30 seconds. Replace cap
cover on the extraction vial. 5. Start timer. Read the result at 10~20 minutes. Do not interpret
the result after 20 minutes. Interpretation of Results NEGATIVE: Only one red band appears in the control region (C), and no band
in the test region (T). The negative result indicates that
there are no Novel coronavirus antigen in the sample or the number
of viral particles is below the detectable range. POSITIVE: Two red bands appear. One red band appears in the control region
(C), and one red band in the test region (T). The shade of color
may vary,but it should be considered positive whenever there is
even a faint band. INVALID: No red band appears in the control region (C). The test is invalid
even if there is a band on test region (T). Insufficient sample
volume or incorrect procedural techniques are the most likely
reasons for control line failure. Review the test procedure and
repeat the test using a new test device Company Information Innovation Biotech (Beijing) Co., Ltd. is a biotechnology company specializing in research, development and manufacturing of advanced medical in-vitro diagnostic (IVD) rapid test kits, medical and laboratory disposal products. We provide one step medical diagnostic rapid test kit based on our INVBIO brand, Product include Fertility Tests, Tumor markers, DOA drug of abuse test, Drug test cup, Urinalysis reagent strip, ELISA kit, Digital alcohol tester, urine analyzer, et. OEM packaging is available, drug of abuse test, troponin I test, alcohol screening saliva test strips, urine strips, elisa kits, microscope slides Our focus is to expand our markets internationally by forming strategic alliances and entering into partnerships with distributors worldwide. We have established an international reputation for excellence in the manufacturing of quality medical and laboratory products. In addition, we have obtained approval licenses ISO13485, FSC certificate, and most of our products get CE mark Our objective is the utmost satisfaction of our clients all around the world by supplying our quality and economical products
Certifications
Contact information Mob/WhatsApp/WeChat/Skype: 008618579609735 Website: www.invbio.com
FAQ Q: What is your payment terms? A: Payment terms: 100% TT before shipment Q: Can you send samples? A: Yes,SAMPLES can be sent for your evaluation.customer pay the freight charge Q: Do you give any discount? |
||||||||||||||||||
| Product Tags: Qualitative Detection Self Rtk Antigen Test Lollipop Self Rtk Antigen Test Rtk Ag Rapid Test Cassette Lollipop |
|
Ce Certified Rtk Coronavirus Antigen Swab Test Self Test 24 months |
|
98% Accuracy Ce Certified Rtk Coronavirus Antigen Swab Test Self Test |
|
Convenient Safe Testing With Covid-19 Ag Saliva Rapid Test Kit Lollipop |
|
Accuracy Reliability Covid-19 Oral Fluid Rapid Antigen Test Kit |
|
Fsc Ce Certified Rtk Coronavirus Antigen Swab Test Self Test |
|
High Accuracy Covid 19 Rapid Test Kit With Germany Bfarm With Ce Approved |